Bagaimana menghitung karakter pada paragraf tertentu?
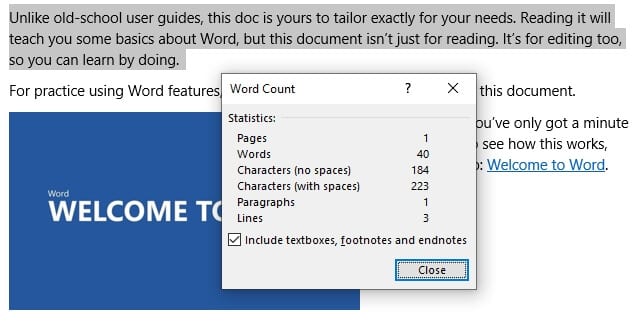
Mudah saja, pertama kamu harus menyeleksi (blockquote) paragraf atau kalimat yang ingin dihitung, kemudian pergi ke menu Review > Word Count. Setelah itu kamu akan melihat jumlah karakter dari teks yang dipilih.






The primary fuels which are burned to release heat and generate steam in boilers are the fossil fuels in the term of coal, fuel oil and natural gas, which represent the remains of plant and animal life that are preserved in the sedimentary rocks. Besides these, industrial wastes like blast furnace gas, coke oven gas, refinery gas, sugar factory refuse (bagasse), saw mill wood dust, rice husk, etc. are also uscd as boiler fuels, often to boost one of the primary fossil fuels. When more than one type of fuel is simultaneously burned to meet the total heating requirement the boiler is said to have a combination firing.
An.iial COAL
Coal is the principal energy source, particularly in India because of its large deposits and availability_ Coal originated from vegetable matter which grew millions of years ago. Trees and plants falling into water decayed and later produced peat bogs. Huge geological upheavals buried these bogs under layers of silt. Subterranean heat, soil pressure and movement of earth’s crust distilled off some of the bog’s moisture and hardened it to form brown coal or lignite. Continuing subterranean activity and metamorphosis produced higher grades .of coal. According to geological order of formation, coal may be of the following types; (1) Peat, (2) Lignite, (3) Subbituminous, (4) Bituminous, (5) Subanthracite, and (6) Anthracite, with increasing percentages of carbon. After anthracite, graphite is formed_ Anthracite contains more than 86% fixed carbon (in amorphous form) and less volatile matter. Volatile matter helps in the ignition of coat. So, it is often difficult to bum anthracite. Bituminous coal is the largest group containing 46-86% of fixed carbon and 20-40% of volatile matter, It can be low-volatiles medium-volatile and high-volatile. The lower the volatility, the higher the heating value. Lignite is the lowest grade of coal containing moisture as high as 30% and high volatile matter, According to ATM (Ametican Society ofTesting and Materials), peat is not regarded as a rank of coal. Peat contains up to 90% moisture and is not attractive as a utility fuel. Rank carries the meaning
160 Power Plant Engineering
of degree of maturation (carbonisation) and is a measure of carbon conicnt iri eit-ii.aL Lignite is considered. to be low rank and anthracite to be high rank,
dal COAL ANALYSIS
There are two types of coal analysis: proximau2 and ultimate, both done on a i-rms per cent basis, Both these types may be based otr, (a) as-received basis, useful for combustion. calculations, (b) dry or moisture free basis., (c) dry mineral-matter-Tree or combustible basis.
4.2.1 Proximate An
The- prirKirnate .no.lyiN indicates thi.2. behaviour of coal when it is heated_ When 1 g sample of coal is subjected to a temperature of about 105 ‘C. for a period of 1 hour. the Loss in weight of the sample gives the moisture content of the coal.
When 1 g sample of coal is placed in a covcrcd platinum crucible and heated to 950 ‘C and maintained al that temperature for about 7 min, there is a loss in weight due to the elimination of moisture and volatile matter. The latter may now be determined since moisture has been calculated from thy previous test. Volatile matter consists of hydrogen and certain hydrogen-earl-31i>> compounds which can bc removed from the coal simply by heating. it.
By subjecting I g sample of coal in an uncovered crucible to a temperature of about 720 “C until the coal is completely burned_ a constant weight is reached, which indicates that there is only ash remaining in the cnicible.. Complete combustion of coal is determined by repeated weighing of the sample.
Fixed carbon is the difference between 1DO% and the sum o f the percentages of moisture, .;:ish and volatile,. matter_ However, this difference does not represent ali the carbon that was in the coal. Some of the carbon may have been in the form o f hydrocarbons which may have been distilled off while determining the volatile matter. It is also possible that some of this fixed carbon may include sulphur, nitrogen and oxygen. So, the proximate analysis of coal gives
VM M.+ A = 100% by mass (4.1)
Thc amount of Vivi indicates whether the coal will burn with a short or king flame and whether it will tend to produce smoke. The more valet& the coal, the more it will smoke_
Figure 4.1 shows the trend in moisture, violatile matter and fixed carbon when expressed on a dry ash-free basis. The general trend with increasing rank is an increase in the heating value and fixed carbon and a corresponding decrease in moisture and VIA. This trend is so pronounced that a classification system based on the fuel ratio (ratio of fixed carbon to volatile matter has been used as a rough indicator of a coal’s rank_
Lower rank coals (lower fuel ratio) are characterised by a greater oxygen con tent, that aids ignition and enhances combustibility and flame stability. High combustibility improves carbon burnout (reduces carbon carryover) and licAlcc;
Fuels and Combustion 164
.
…..
I ‘i
II.
NEWIIIISC.-….NC –
–
i
.,… ‘ I I
__
snou I …-
4.!
= D .,…
0 wt,
..
I !um1141
, . 5 2,r
r ,.. M •111
2
.a.
_
irl’iqu Rur tleCLIt % nocr 31V1.0 . , I
- . – .- L..
4.- 4..
– –
..-
0 0 – u C
1.1ui 1 Subbit Suhhi !qqns Mil ”’. -.r. • 8 IQ
.L.0 -0 ‘= .’ -0
Eir.1 er 4-na oli
.-.
e-I
Moislurc: I JVolaii.lc maticr Fixed carbon
Fig 4.1 Coal rank compared with proximate analysis
boiler efficiency and for pulverligd LORI -fired units, this allows the coal to be ground to a coarser size. Low rank coals (high moisture contcni) produce a “self-puiverization” of the coal particles during combustion_ As the inherent moisture in the pore stnicture of the coal is, heated and expands rapidly, its volume increases (as water flashes to steam at atmospheric pressure, the volume expansion is 1600 to 1), thus fragmenting the coil panicles. This exposes more surface area for combustion.
4.2.2 Ultimate Analysis
The ultimate amaly5is gives the chemical elements that comprise the coal substance, together with ash and moisture. The coal substance consists of organic compounds of carbon, hydrogen, and oxygen derived from the original vegetable matter. The analysis shows the following components on mass basis: carbon (C), hydrogen (H). oxygen (0), nitrogen (N), sulphur (S), moisture (M) and ash (A). Therefore,
C-1-H-1-01-N+S-1-1V1+A11140%byrnass (42)
The dry and ash free an a lysis on combustible basis is. obtained on dividing C, H,
0. N and S by the fraction (1 M. + A
100
4.2.3 Coal Properties
There are certain properties of coal which are important in power plant applications_ They are swelling index, grinctabiiity, weatherability, sulphur comm.,. heating value and ash softenint,, temperature.
Swelling index Some types of coal during and after release of volatile
matter become soft and pasty. and form agglomerates_ These are called caking
162 Power Mane Engineering
coat. In a fixed bed, such as a tra.voll i ng grate stoker, the coal must not cake a it burns. The consequent agglomeration disturbs greatly the availability of air and go the coal does not completely burn yielding low combustion. efficiency. Coal that does riot cake is ea! ledfree-burning coal _ It breaks apart during combustion exposing large surface area to the air, thus enhancing the combustion process_ Caking CO319 are used to produce coke by heating in a coke oven in the absence of air, with the volatile matter driven off. Coal devoid of volatile miter is called coke, which is largely needed in steel plants. A qualitative evaluation method, called the swelling index, has been devised to determine the extent of caking of a coal. A free-burning coal has a high value of swelling, index, which indicates that it somewhat expands in volume during combustion. Wh.on modern ptilvrized coal burners are uNed, the swelling property of coal is however, of leNs i mportance.
Grindability Grindability is often an important criterion for selecting a coal. This property of coal is measured by the standard grhtdahility index, which is inversely proportional to the power required to grind the coal to a specified particle size for burnii.g. Grindnbilitry of a standard coal is defined as 100. If the coal selected for use at a power plant has ii grindability index of 50, it would require twice the grindiniz, power of the standard coal to produec a specified parLicic sire.
Weathera.bility It is a measure of how well coal can be stockpilect for long periods of time without crumbling to piucc5. Modern power plants normally sto.ckpilc 60 to 90 days’ supply of coal in a large pile near the power plant. The coal unloaded from wagons is packed in a long trapezoidal pile. Excessive crumbling or weathering of the coal due to climatic conditions tna y result in small particles of coal which can be dispersed by wind or rain.
Sulphur content Sulphur content in coal is combustible and generates some energy by it5t oxidation to 502. Sulphur dioxide is a major source of atmospheric pollution, Their is an environmental mgulation ri 50 emission, The operating cost of SO removal equipment need be considered while selecting a coal with high sulphur content.
Heating value The heating value or calorific value of coal is a property of fundamental importance_ It may be determined on as-received, dry, or dryand-ash-free basis. It is the heat tra.nsferred when the products of complete ecanbustion of a sample of coal (o other fuel) arc cooled to the in temperature of air and fuel, It is normally determined in a standard test in a bomb calorimeter, where a coal sample of known mass is burnt with pure oxygen supply completely in a stainless steel bomb or vessel surrounded v a known mass of water., and the rise in water temperature is noted. Two different heating values are cited for coal. The higher heating value (H1-EV) assumes that the water vapour in the products condenses and thus includes the 1a tint bad of vapr3rization of the water yap-our formed by combustion. The lower heating value 0…H. V.) assumes that OH.; water vapour fonnocl by combustion leaves as vapour itself. Therefore,
Lels and Combustion 163
LHV= HHV — itrg (4.3)
where mw is the mass of water vapour rormed given by
in„.= + 9ff + A ti.A 0_4)
where M and H are the mass fractions of moisture and hydrogen in the coals
is the specific humidity of atmospheric air and WA is the actual amount of air supplied. per of coal_ For energy balance and efficiency calculations of steam
generators, HHV of fuel is considered in the USA_ whacas LHV is tile. stand:L-1rd used in European praotice,
if the ultimate an.alysis is known, the WV of anthracite and bituminous coals can be determined approximately by wing Dulong and Petit formula ELS given below
HFib= 33,83 C± 144-45 (H —0 ) 9_1g 5. in Ntlikg (45)
where C, H, 0 and S are mass fractions of carbon, hydrogen, oxygen and sulphur in coal. Assuming the latent heat of vaporization hirg at the partial pressure or water vapour in the combustion products as 2.395 Mi/kg, the liy..vor heating value of coal from El_ (4,3) is given by
LI-IV = 1-11-IV — “Ong (4.6)
For lower-rank fuels, E.q.. (4.5) usually underestimates the HH.V.
Ash softening temperature The ash softening temperature is the temperature at which the ash softens and becomes plastic. This is somewha( below the melting point of ash. The design of the steam generator greatly depends on die ash softening temperature (as,t,) of the coal. If the furnace temperature is higher than the aisrt., all the ash will melt und would come out of the furnace bottom. continuously as molten slag_ For a furnace that would discharge ash in the solid form,, a high ash softening temperature would he required. A stoker furnace must use coal with a high a.s.t., other…vise clinkers would be formed. Clink ers. which are large masses offs ash, cause troubles in discharge and also make combustion inefficient,
Spontaneous combustion Combustion (oxidation) of coal can take pi= rapidly as in a furnace or slowly on a stockpile. If it takes place slowly, there is a degradation or loss of energy content and hence in the value of fuel, The factors which influence spontaneous combustion and which can lead to a big fire, are the following.
(a)Rank of coal. low rank coals are more susceptible because oftheir higher porosity.
(b)Amount of surface area exposed to air,
(e) Ambient temperature, with high solar insolation aiding it,
(ci) Oxygen content of coal.
(e)Free moisture in coal
(f)Configuration of the coal stockpile; steep conical p i I with coarse coal at the edges and fines near the top are more susceptible because they
&pel.i Power Rant „e ngi neeri ng
promote natural convection (chimney effect) and good air flow through the pi lc to support combustion a it develops..
To prevent spontaneous combustion, it is important to maintain a dry pile and compaction at regular intervals.
42.1 FUEL OIL
Petroleum is he to have been formed during past geological ages from
decayed marine life, both vegetable and animal. Dead marine animals and vegetable matter accumulated for millions of years ultimately got transformed into oil, mainly in sedimentary rocks, by pressure and heat. Oil deposits accumulated. in the rocks and sands below the earth’s cram Oil generally has a body of water below and pressurized natural gas above. Fairly thick and dense earin strata (caprock) cover most && sits preventing seepagc (Fig. 4.1a). Oil wells dallied through this layer penetrate the deposits. The pressure forces the
as and oil to the surface. Aficr the pressure has diminished, the oil MUM. be pumml.